Cation Exchange Capacity (CEC)
Determination of sorption and swelling capacities of special clay minerals
The cation exchange capacity (CEC) is a scientific parameter used to determine special properties of clay samples such as sorption or swelling capacities. The analytical method we use in our laboratory is based on the principle of exchanging the interlayer cations of clay minerals with the Cu-triethylenetetramine complex ("Cu-trien"). Photometric analysis of the exchanged suspension of Cu-triene solution and sample powder allows the quantification of the exchanged molar amount of Cu-trien molecules. To ensure the highest possible data quality for our customers, standardised pH corrections are included in our evaluation. Thanks to these corrections, sample-specific influences on the CEC can be reduced to a minimum.
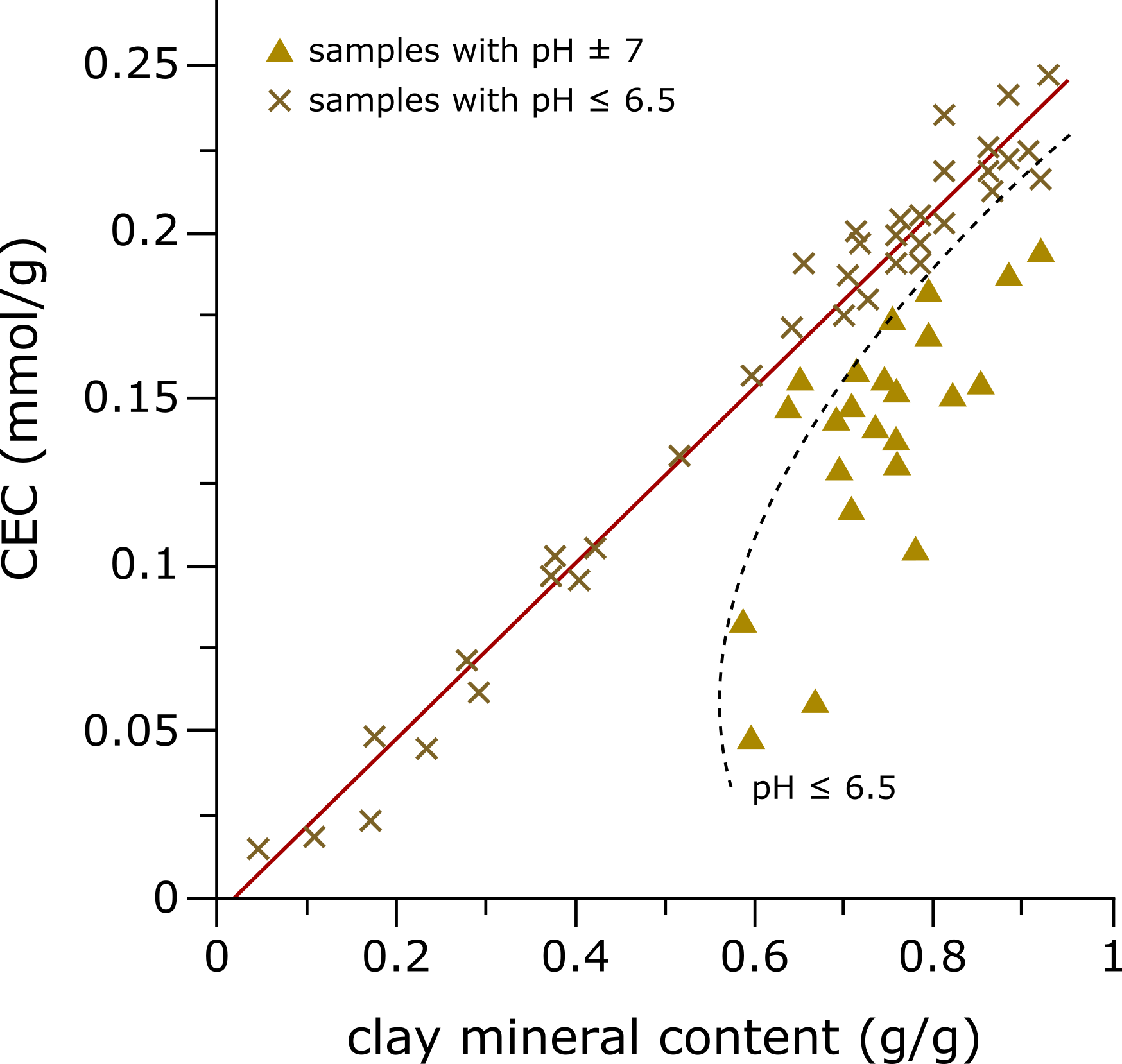
Figure 1: Correlation between the cation exchange capacity (CEC) in "mmol/g" and the total clay mineral content of a series of sediment samples in "g/g". In this figure, one can see the linear relationship between the CEC and the clay mineral content under neutral pH conditions (red line). In addition, this figure illustrates the effect of lower pH values when measuring CECs, resulting in significantly underestimated CEC values (black dashed line). The reason for this effect is the protonation by H+ ions of the negatively charged clay mineral surfaces. For this reason, we always carry out a pH correction of the measured values for all CEC measurements.
Applications
The cation exchange capacity is used as a scientific parameter in many fields:
- Determination of smectite contents in quality assurance in clay mining
- For the assessment of damage to buildings caused by the soil of the building site
- Assessment of the formation damage potential of clay-containing rocks
- As a correction factor for evaluation of well log data in the oil & gas industry
- Characterisation of special clay raw materials for landfill sites and nuclear waste disposal